The phenomenon of change of matter from one state to another state and back to original sate, by alternating the condition of temprature or pressure , is called interconversion of matter.
Interconversion of states of matter can be achieved
By changing the temprature
By changing the pressure
1. BY CHANGING THE TEMPERATURE:-
EFFECT OF CHANGE OF TEMPRATURE ON STATES
When thermal energy is added to a substance, its temperature increases, which can change its state from solid to liquid (melting), liquid to gas (vaporization), or solid to gas (sublimation). ... Decreasing pressure can cause it to vaporize. For some types of rock, decreasing pressure can also cause them to melt.
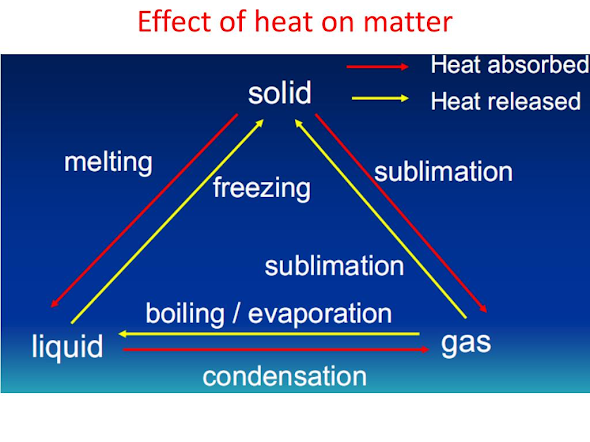
👉SOLID TO LIQUID CHANGE: MELTING
The process in which a solid changes into a liquid on heating is called melting or fusion.
👉The temprature at which a solid changes into liquid at atmospheric pressure is called melting point of the substance.
👉 Higher the melting point,stronger are the forces of attraction between the particles.
example- The most common example is solid ice turning into liquid water. This process is better known as melting, or heat of fusion, and results in the molecules within the substance becoming less organized.
The process in which a liquid substance changes into a gas on heating is called Boiling or vaporization.
🖊 The temperature at which a liquid boils and changes into gas at atmospheric pressure is called boiling point of the liquid.
🖋 Impurities increase the boiling point of liquids.
🖋 If pressure is increased, the boiling point increases. Boiling point of water is taken as 373k at 1 atm pressure.
🖋 Heigher the boiling point, stronger are the forces of attraction between the particles.
example- A good example of boiling is seen when water is heated until it forms steam. The boiling point of fresh water at sea level is 212°F (100°C). The bubbles that form in the water contain the vapor phase of water, which is steam.
👉GAS TO LIQUID CHANGE : CONDENSATION
✏ The process of changing a gas to liquid on cooling is called condensation.
🖊 Condensation is the reverse the vaporization.
example-
- Morning Dew on the Grass. ...
- Clouds in the Sky. ...
- Rain Falling Down. ...
👉 LIQUID TO SOLID CHANGE: FREEZING
💭The process of changing a liquid into solid by cooling is called freezing.
💭 Freezing is also called solidification and is reverse of melting.
💭 The temprature at which a liquid freezes to became a solid at atmospheric pressure is called the freezing point.
💭 Impurities lower the freezing point of liquids.
example - The most common example of freezing, which is observed every day, is the formation of ice cubes in ice-tray when water is kept in the freezer for some time.
👉 SUBLIMATION ( SOLID TO GAS OR GAS TO SOLID ) :
The process of change of a solid state directly to gaseous state on heating and vice-versa on cooling without passing through the intervening liquid state is called sublimation.
Examples of Sublimation
The best example of sublimation is dry ice which is a frozen form of carbon dioxide. When dry ice gets exposed to air, dry ice directly changes its phase from solid-state to gaseous state which is visible as fog. Frozen carbon dioxide in its gaseous state is more stable than in its solid-state.SOME MORE EXAMPLES- CAMPHOR, AMMONIUM CHLORIDE, NEPTHALENE, IODINE,etc.
 |
| Ammonium chaloride |
 |
| IODINE |
 |
| Nephthalene |
 |
Camphor👉 APPLICATIONS OF SUBLIMATION:-
PLEASE SUBSCRIBE MY YOUTUBE CHANNEL:-https://www.youtube.com/channel/UCIIEve3CJeYCn6_yVxAlJGQ |






